This position has been filled and is no longer available.
A 3-year PhD studentship is available in the group of Marcel Utz, supported by the Institute for Life Sciences (IfLS). The successful candidate should have a good degree in chemistry, biochemistry, physical chemistry, or biophysics and a special interest in nuclear magnetic resonance spectroscopy and/or microfluidic systems.
 Nuclear magnetic resonance (NMR) spectroscopy is one of the most powerful analytical tools available to study the dynamics, structure, and interactions of proteins. In addition to detailed 3D structural information, NMR is particularly apt at providing information on dynamic processes, as well as binding interactions with peptides and other substrates. NMR is inherently non-invasive. In sharp contrast to other techniques such as x-ray diffraction, which require crystalline samples, high vacuum, and employ intense ionising radiation, NMR allows the study of proteins (and other systems) under very mild and realistic conditions, in some cases even directly in-vivo. The main aim of the present project is to bring this advantage to bear in the context of microfluidic lab-on-a chip devices.
Nuclear magnetic resonance (NMR) spectroscopy is one of the most powerful analytical tools available to study the dynamics, structure, and interactions of proteins. In addition to detailed 3D structural information, NMR is particularly apt at providing information on dynamic processes, as well as binding interactions with peptides and other substrates. NMR is inherently non-invasive. In sharp contrast to other techniques such as x-ray diffraction, which require crystalline samples, high vacuum, and employ intense ionising radiation, NMR allows the study of proteins (and other systems) under very mild and realistic conditions, in some cases even directly in-vivo. The main aim of the present project is to bring this advantage to bear in the context of microfluidic lab-on-a chip devices.
 The ability to perform meaningful NMR studies of protein systems on a chip would open interesting possibilities. For example, binding studies of a protein with an entire library of peptides could be efficiently integrated on a chip, offering considerable savings of cost and time.
The ability to perform meaningful NMR studies of protein systems on a chip would open interesting possibilities. For example, binding studies of a protein with an entire library of peptides could be efficiently integrated on a chip, offering considerable savings of cost and time.
Protein NMR almost universally relies on isotopically labelled samples. Most commonly, proteins are expressed in E. coli cultures fed with universally 15N and 13C labelled amino acids. This process is costly, and the ability to work with very small samples is therefore inherently attractive. Moreover, many proteins of interest are difficult to express in large quantities.
The present project is designed to explore quantitatively the technical challenges that need to be overcome to make microfluidic protein NMR possible. With the specific aim of carrying out a repeated dilution protein binding study, it is designed

to produce a convincing demonstration of the potential of this approach.
The magnetic resonance research section at the University of Southampton, UK, has access to a wide variety of state-of-the-art magnetic resonance instruments including NMR and EPR spectrometers, as well as hyperpolarization technologies such as dynamic nuclear polarization (DNP) and parahydrogen-induced polarization (PHIP). In addition, Southampton provides an ideal environment for microfluidic technology, with several internationally renowned research groups active in this field.
The project welcomes applicants from the EU/UK who have or expect to obtain a first class degree in Chemistry, Physics or allied subjects/relevant disciplines. Funding will cover fees and a living allowance in line with RCUK guidelines (£14553 for 2017/2018).
Applications for a PhD in Chemistry should be submitted online at https://studentrecords.soton.ac.uk
Please ensure you select the academic session 2018-2019 in the academic year field and click on the Research radio button. Enter Chemistry in the search text field.
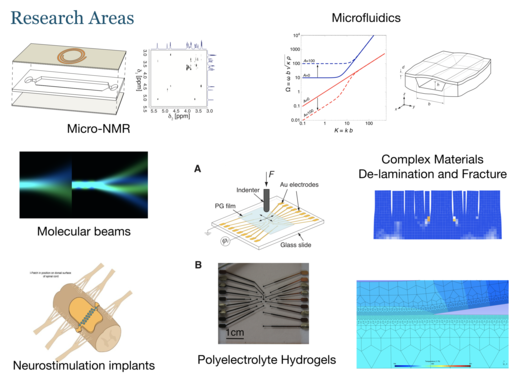 Our research mainly concerns the study of live systems (cells, tissues, and small animals) by nuclear magnetic resonance. We are developing miniaturised “Lab-on-a-chip” devices for the culture of such biological systems, and integrate them with in-situ observation by nuclear magnetic resonance. This combination of microfluidic culture technology with advanced nuclear magnetic resonance techniques provides unique insight into the metabolic processes of life. Our work is motivated by applications across the life sciences, in particular in drug discovery and safety testing, and in the development of disease models.
Our research mainly concerns the study of live systems (cells, tissues, and small animals) by nuclear magnetic resonance. We are developing miniaturised “Lab-on-a-chip” devices for the culture of such biological systems, and integrate them with in-situ observation by nuclear magnetic resonance. This combination of microfluidic culture technology with advanced nuclear magnetic resonance techniques provides unique insight into the metabolic processes of life. Our work is motivated by applications across the life sciences, in particular in drug discovery and safety testing, and in the development of disease models.